What is Temperature?
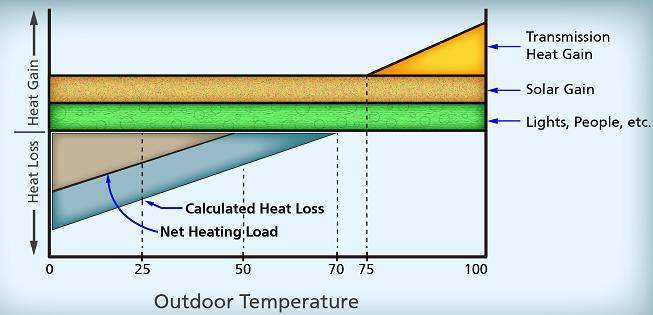
Temperature and heat are not the same thing. This is an important distinction that needs to be made right off the bat. Heat we measure in total heat quantity to acquire total heat content. Temperature is the measure of average molecular velocity within a substance. Another way of saying it is a measure of kinetic energy within a substance. The reason why the word average is used is because some molecules are moving faster within a substance and some are moving slower. If you average out the energy within a substance, let’s say a glass of water. If you put a thermometer into a glass and it reads 80 degrees, that tells you the average molecular velocity within that substance. If you have another cup of water that measures 60 degrees, that would mean that the average molecular velocity in the 80 degree cup of water is higher. Which also means that the molecules in the 80 degree cup are moving faster than the 60 degree cup. If you take the 80 degree cup of water and compare it to an 80 degree pool they would have the same molecular velocity. This also means that they have thermal equilibrium. What that means is you would not lose heat energy if you were to put the glass and pool water together. That does not mean that they have the same amount of heat in them. The swimming pool has more heat because it has more molecules. (refer to the temperature measure graph below for a temperature measuring example)
Cold is the absence of heat. We do not measure the amount of cold like we measure amounts of heat. However we do measure a point of cold. Where we measure the point of cold is absolute zero.It’s the theoretical point at which all molecular movement stops. With heat there is no maximum amount that can be measured.
The biggest thing we can do with temperature is compare molecular velocities to each other in order to see which direction heat will move. For example, when you are saying that you are cold you are essentially saying the rate at which heat is leaving my body is making me uncomfortable. Water for example has a greater density of molecules and transfers heat faster. So water that is colder is going to transfer heat out of your body at a faster rate. Water that is warmer is going to transfer heat out of your body at a lesser rate. Heat transfer is what makes an individual feel hot or cold. If heat is moving into your body that’s hot. If heat is moving out of the body that’s cold. When there are greater differential temperatures heat moves quicker.
Total heat content of something is generally measured in BTU’s. A btu is defined by the total amount of heat required to change one pound of water by 1 degree fahrenheit. It is about the amount of heat coming off a match.
Temperature Scale
Absolute zero on the fahrenheit scale is -460 degrees. In the celsius scale it’s equivalent to -273 degrees. The kelvin scale is the Celsius version of the temperature scale. The Rankine scale is the fahrenheit version of the temperature scale in HVAC. They both start at absolute zero, and have no negative numbers. (refer to the graph below)
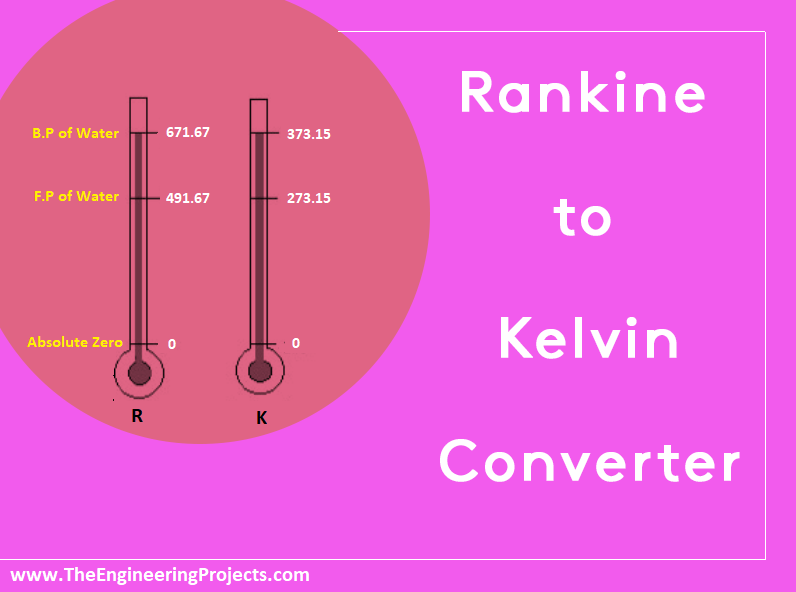
The absolute zero for Rankine starts at -460 degrees fahrenheit. The absolute zero for Kelvin starts at -273 degrees Celcius. For all your HVAC needs, feel free to contact one of our techs at (256)-585-2550.